미국 시장을 겨냥한 K-뷰티 SPF, 기획 중이신가요?
자외선 차단제, 여드름 케어, 피부 보호제 등은 FDA에서 OTC 의약품으로 분류되어 엄격한 규제 준수가 필수적입니다.
까다로운 OTC 제품, 검증된 전문 제조 시설이 해답입니다.
CTKCLIP의 OTC 제품은 글로벌 K-뷰티 혁신을 선도하는 미국 제조 자회사, CTK OTC Laboratories 에서 생산됩니다.
캘리포니아 남부에 위치한 최첨단 시설에서 퍼스널 케어, 색조 화장품, OTC 제품을 직접 개발하고 생산하며, 한국의 정밀함과 현지의 속도를 결합한 최적의 솔루션을 제공합니다.
CTKCLIP OTC가 특별한 이유

신뢰할 수 있는 미국 자체 제조 시설
FDA에서 감사 시 의약품 기준으로 진행되기 때문에 까다로운 검사 조건을 통과할 수 있는 믿을 수 있는 제조사와 함께 해야 합니다. CTKCLIP은 수년간의 OTC 제조 경험을 보유한 인증된 미국 제조 시설에서 생산됩니다.

개발 기간, 지치지 않게. OTC White Label
남들보다 한발 앞서 빠르게 런칭해 보세요.

스타트업도 부담 없는 MOQ
경쟁력 있는 최소 주문 수량(MOQ)으로 미국 시장 진출에 최적화된 솔루션을 제안합니다.
제일 중요한 건 안정성과 효율성
OTC monograph를 준수하여 안정성과 효율성이 입증된 활성 성분들로 개발합니다.
어떤 OTC 제품을 개발 할 수 있나요?
미국에서 OTC로 분류되는 제품들로 문의를 남기고 개발을 시작해 보세요.

자외선 차단제 SUNSCREEN
미국에서 자외선 차단제는 화장품이 아닌 의약품으로 규정하고 있는 OTC 제품입니다. 베이스 메이크업(파운데이션, CC크림)도 SPF를 포함하는 경우, OTC로 분리됩니다.
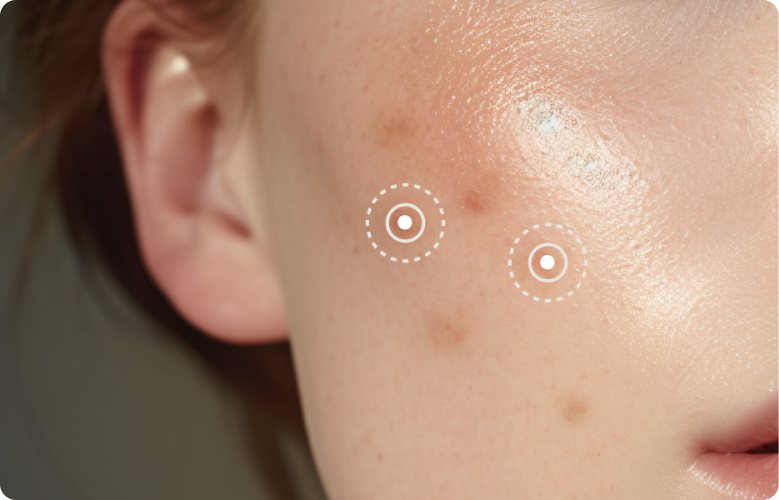
여드름 제품 ACNE
여드름 유효성분(Acne medication active ingredient)을 일정량 함유한 제품입니다.

피부 보호제 SKIN PROTECTANT
자극으로부터 손상된 피부를 일시적으로 보호하고 진정하는데 도움을 주는 제품입니다.

